Medical Device Testing Service
Established in 1963, STC is an independent, not-for-profit testing, inspection and certification organization headquartered in Hong Kong, offering one-stop professional conformity assessment service for customers around the world to get access to the global markets.
As an organization with global network, not only has STC set up testing facilities and customer service offices in China’s major cities such as Dongguan, Shenzhen, Zhongshan, Shanghai and Changzhou, but also in countries like Germany, Italy, USA, Vietnam and Japan. Our world-class, internationally accredited testing facilities can meet the needs from a wide range of industries.
We have been continuously expanding our service scope. After years of development, STC has established a pre-clinical medical device R&D test platform that meets international standards, and established a series of full-featured laboratories such as passive medical device laboratories and active medical device laboratories. To meet the pre-clinical R&D testing needs of medical device manufacturers, providing one-stop services, including chemical characterization, biocompatibility testing, large animal testing, microbiological testing, safety and performance verification, and electromagnetic compatibility testing. STC Medical Device Laboratories has been recognized by a wide range of domestic and international organizations, including IECEE, CE, DAkkS, FDA, NEMKO, INMETRO, CNAS, CMA, etc., and became the first Chinese OECD member of the GLP regulatory agency EMA (Entidad Mexicana de Acreditacion, ac) Review of certified medical device GLP laboratories.
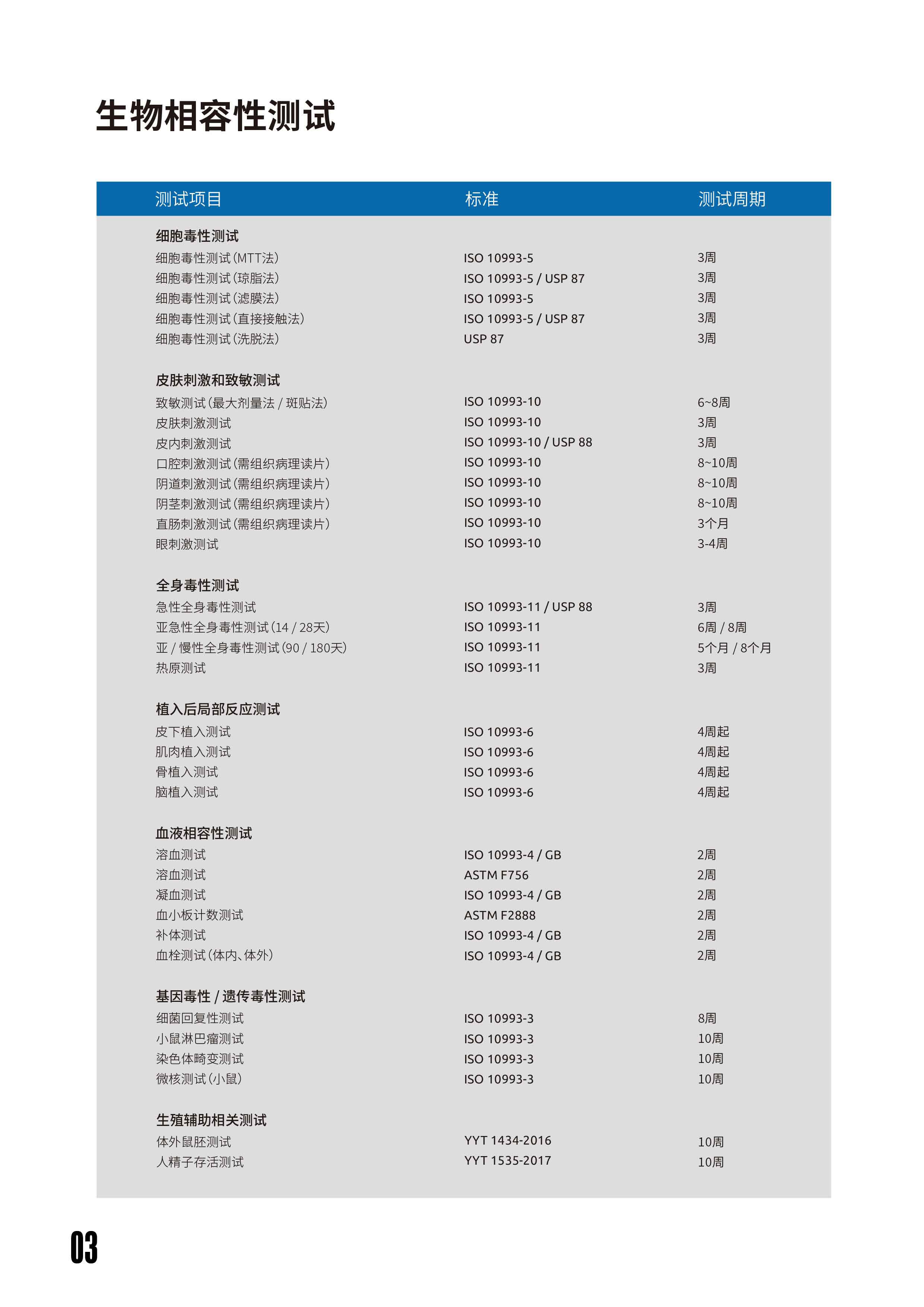
- Biocompatibility Test
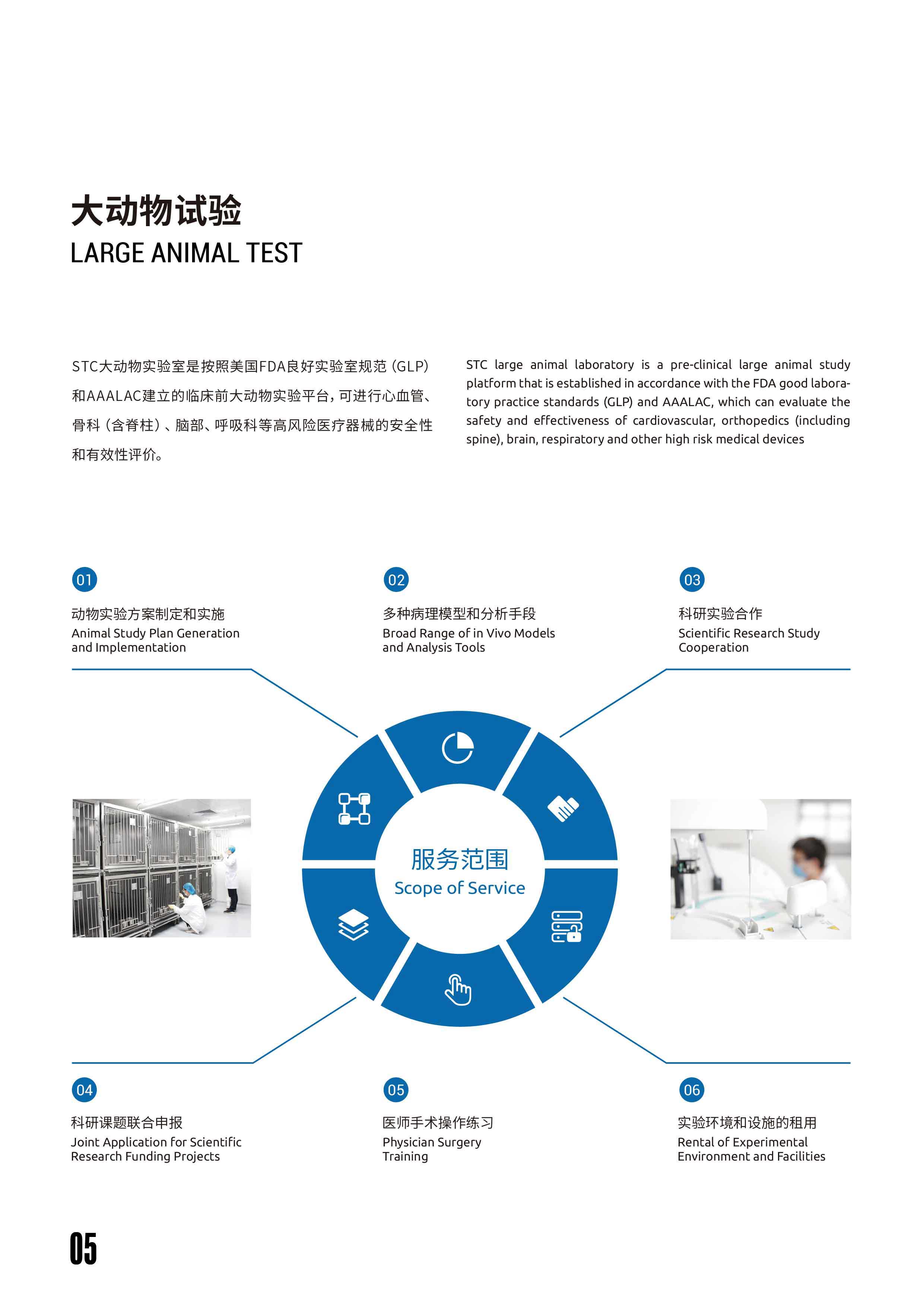
- Large Animal Test
- Microbiological Test
- Chemical Test
- Safety and Performance Test
- Electromagnetic Compatibility Test
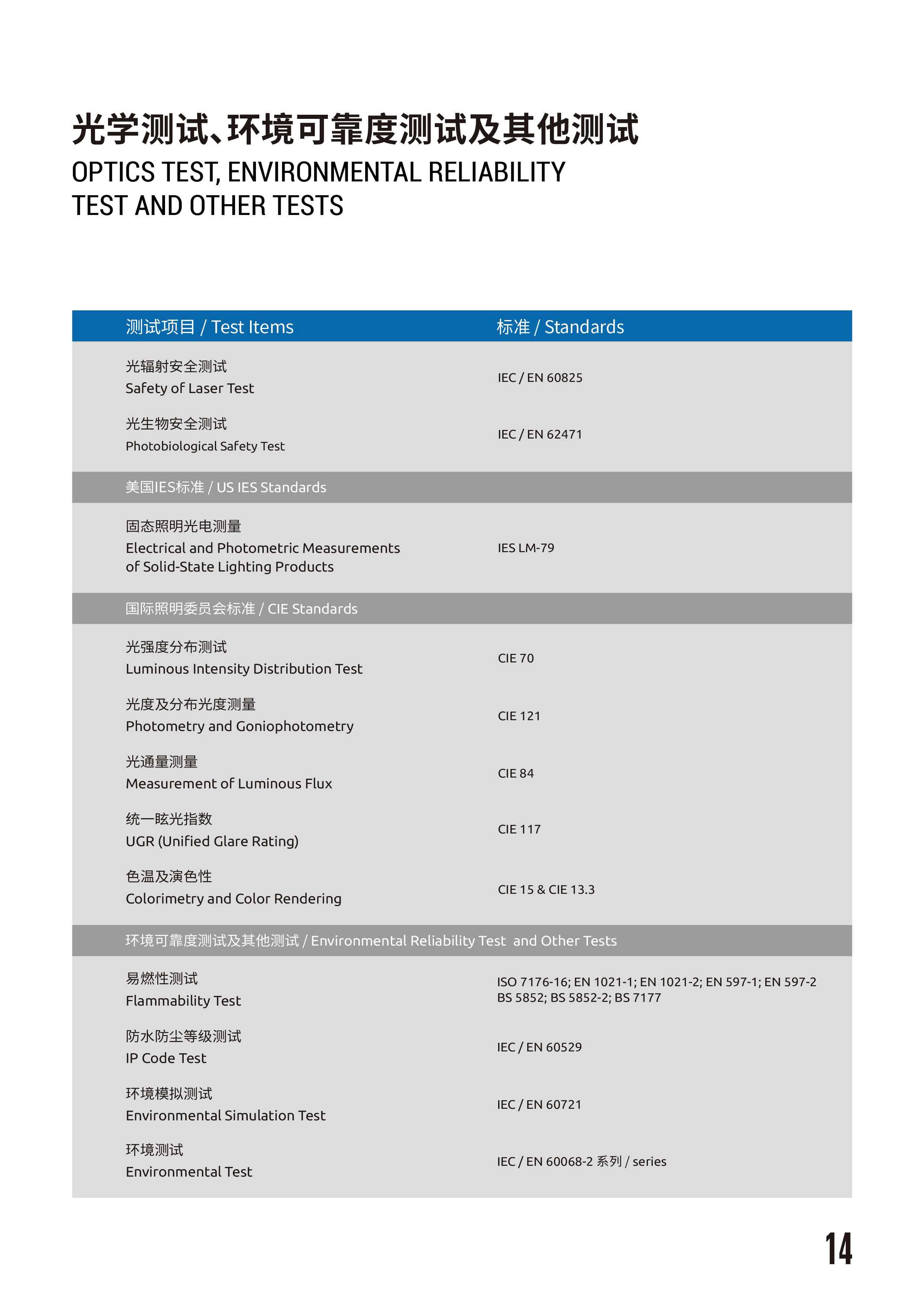
- Optics Test, Environmental Reliability Test and Other Tests